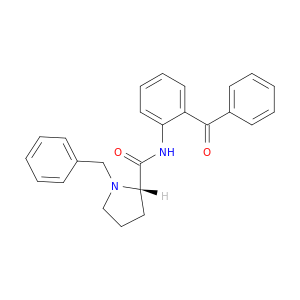
(S)-(-)-2-(N-Benzylprolyl)aminobenzophenone
| Title | Journal |
|---|---|
| (SP-4-4)-[Hydrogen N-({2-[(2S)-1-benzyl-pyrrolidine-2-carboxamido]phen-yl}(phen-yl)methyl-ene)-l-glutamato(2-)]nickel(II). | Acta crystallographica. Section E, Structure reports online 20090401 |
| Characterization of Ni(II) complexes of Schiff bases of amino acids and (S)-N-(2-benzoylphenyl)-1-benzylpyrrolidine-2-carboxamide using ion trap and QqTOF electrospray ionization tandem mass spectrometry. | Journal of mass spectrometry : JMS 20080901 |
| No carrier added synthesis of O-(2'-[18F]fluoroethyl)-L-tyrosine via a novel type of chiral enantiomerically pure precursor, NiII complex of a (S)-tyrosine Schiff base. | Bioorganic & medicinal chemistry 20080501 |
| Off-line combination of reversed-phase liquid chromatography and laser desorption/ionization time-of-flight mass spectrometry with seamless post-source decay fragment ion analysis for characterization of square-planar nickel(II) complexes. | Journal of mass spectrometry : JMS 20060401 |
| Enantioselectivity in Ni(II) Schiff-base complexes derived from amino-acids and (S)-o-N-(N-benzylprolyl)aminobenzophenone: molecular structure of several chiral Ni(II) Schiff-base complexes, circular dichroism and molecular mechanics studies. | Dalton transactions (Cambridge, England : 2003) 20050707 |
| Improved synthesis of proline-derived Ni(II) complexes of glycine: versatile chiral equivalents of nucleophilic glycine for general asymmetric synthesis of alpha-amino acids. | The Journal of organic chemistry 20030905 |