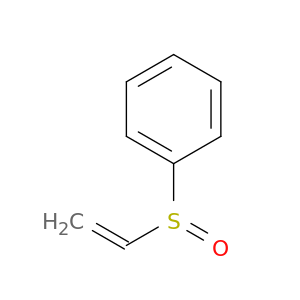
Phenyl vinyl sulfoxide
| Title | Journal |
|---|---|
| Probing the formation of bicyclo[4.2.0]octan-1-ols. | The Journal of organic chemistry 20040820 |
| Diels-Alder reactions of 4-triflyloxy-2,6,6-trimethyl-2,4-cyclohexadienone. An expedient methodology for the synthesis of bicyclo[2.2.2]oct-5-en-2-ones and bicyclo[2.2.2]octa-5,7-dien-2-ones. | The Journal of organic chemistry 20040123 |
| The formation of bicyclo[n.2.0]alkan-1-ols from the reaction of the lithium enolates of simple ketones and phenyl vinyl sulfoxide. | Organic & biomolecular chemistry 20030421 |
| Lead discovery of alpha,beta-unsaturated sulfones from a combinatorial library as inhibitors of inducible VCAM-1 expression. | Bioorganic & medicinal chemistry letters 20030224 |