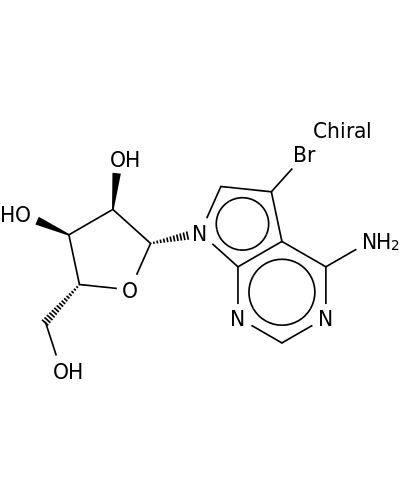
5-Bromo-7-β-D-ribofuranosyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
| Title | Journal |
|---|---|
| A CoMSIA study on the adenosine kinase inhibition of pyrrolo[2,3-d]pyrimidine nucleoside analogues. | Bioorganic & medicinal chemistry 20080501 |
| Biochemical and biological properties of 5-bromotubercidin: differential effects on cellular DNA-directed and viral RNA-directed RNA synthesis. | Bioorganic & medicinal chemistry 20080201 |
| A TOPS-MODE approach to predict adenosine kinase inhibition. | Bioorganic & medicinal chemistry letters 20040621 |
| QSAR study on adenosine kinase inhibition of pyrrolo[2,3-d]pyrimidine nucleoside analogues using the hansch approach. | Bioorganic & medicinal chemistry letters 20020325 |
| Synthesis and antiviral activity of some new S-adenosyl-L-homocysteine derivatives. | Journal of medicinal chemistry 19921127 |
| Synthesis, antiproliferative, and antiviral activity of certain 4-aminopyrrolo[2,3-d]pyridazine nucleosides: an entry into a novel series of adenosine analogues. | Journal of medicinal chemistry 19920207 |
| Design, synthesis, and studies on the structure activity relationships of certain pyrrolo[2,3-d]pyrimidine nucleosides and structurally related analogs as potential antineoplastic and antiviral agents. | Farmaco (Societa chimica italiana : 1989) 19910101 |
| Synthesis, antiproliferative, and antiviral activity of certain 4-substituted and 4,5-disubstituted 7-[(1,3-dihydroxy-2-propoxy)methyl]pyrrolo[2,3-d]pyrimidines. | Journal of medicinal chemistry 19900701 |
| Synthesis and antiviral activity of certain 4- and 4,5-disubstituted 7-[(2-hydroxyethoxy)methyl]pyrrolo[2,3-d]pyrimidines. | Journal of medicinal chemistry 19881101 |
| Structure-activity relationship of novel oligopeptide antiviral and antitumor agents related to netropsin and distamycin. | Journal of medicinal chemistry 19860701 |
| Comparative efficacy of broad-spectrum antiviral agents as inhibitors of rotavirus replication in vitro. | Antiviral research 19860101 |
| Broad-spectrum antiviral activity of adenosine analogues. | Antiviral research 19840601 |
| Antiviral activity of C-5 substituted tubercidin analogues. | Journal of medicinal chemistry 19840301 |