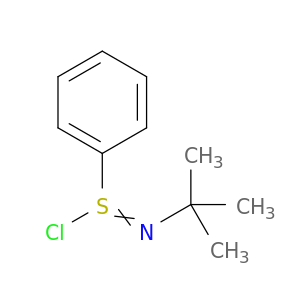
N-tert-Butylbenzenesulfinimidoyl chloride
| Title | Journal |
|---|---|
| Direct and efficient one-pot preparation of ketones from aldehydes using N-tert-butylphenylsulfinimidoyl chloride. | Organic letters 20061026 |
| Oxidative Mannich reaction of N-carbobenzyloxy amines with 1,3-dicarbonyl compounds. | Organic letters 20060914 |
| Imino sulfinamidines: synthesis and coordination chemistry of a novel class of chiral bidentate ligands. | Inorganic chemistry 20060417 |
| Highly efficient methods for the one-pot synthesis of beta-substituted enones. | Organic & biomolecular chemistry 20060107 |
| One-pot beta-substitution of enones with alkyl groups to beta-alkyl enones. | Chemical communications (Cambridge, England) 20050514 |