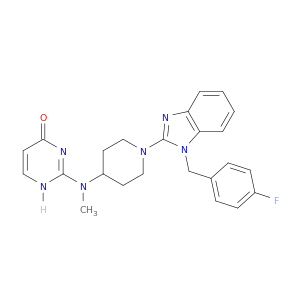
2-[(1-{1-[(4-fluorophenyl)methyl]-1H-1,3-benzodiazol-2-yl}piperidin-4-yl)(methyl)amino]-3,4-dihydropyrimidin-4-one
| Title | Journal |
|---|---|
| Why are most phospholipidosis inducers also hERG blockers? | Archives of toxicology 20171201 |
| Trace analysis of three antihistamines in human urine by on-line single drop liquid-liquid-liquid microextraction coupled to sweeping micellar electrokinetic chromatography and its application to pharmacokinetic study. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20120901 |
| Cetirizine and loratadine: minimal risk of QT prolongation. | Prescrire international 20100201 |
| Reversible anti-settlement activity against Amphibalanus (=Balanus) amphitrite, Bugula neritina, and Hydroides elegans by a nontoxic pharmaceutical compound, mizolastine. | Biofouling 20091101 |
| [Efficacy and safety of Mizolastine in the treatment of perennial allergic rhinitis]. | Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery 20070601 |
| Development of a CZE method for the determination of mizolastine and its impurities in pharmaceutical preparations using response surface methodology. | Electrophoresis 20070201 |
| Common pharmacophores for uncharged human ether-a-go-go-related gene (hERG) blockers. | Journal of medicinal chemistry 20061116 |
| Allergic reactions due to mizolastine. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20060801 |
| [Mizolastine improves the quality of life in patients with perennial allergic rhinitis: a randomized, double blind, controlled study]. | Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery 20060701 |
| Influence of mizolastine on antigen-induced activation of signalling pathways in murine mast cells. | Clinical and experimental dermatology 20060301 |
| [Inhibitory effects of mizolastine on substance P-induced production of leukotriene B4 and interleukin 5 in mouse skin]. | Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Medical sciences 20060301 |
| Hypersensitivity reaction to mizolastine: study of cross reactions. | Journal of investigational allergology & clinical immunology 20060101 |
| The effect of mizolastine on expression of vascular endothelial cell growth factor, tumour necrosis factor-alpha and keratinocyte-derived chemokine in murine mast cells, compared with dexamethasone and loratadine. | Clinical and experimental dermatology 20050301 |
| [Comparative activity of antihistamines on area under dose-response curve from histamine-induced wheal and flare responses in human skin]. | Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae 20041201 |
| Anaphylaxis to mizolastine. | The Journal of allergy and clinical immunology 20041001 |
| Comparison of pharmacokinetics and metabolism of desloratadine, fexofenadine, levocetirizine and mizolastine in humans. | Fundamental & clinical pharmacology 20040801 |
| [Clinical examination of mizolastine in the seasonal allergic rhinitis]. | Lin chuang er bi yan hou ke za zhi = Journal of clinical otorhinolaryngology 20040801 |
| Mizolastine and fexofenadine modulate cytokine pattern after nasal allergen challenge. | European annals of allergy and clinical immunology 20040401 |
| Differential modulation of mediator release from human basophils and mast cells by mizolastine. | Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 20040201 |
| Prediction of hERG potassium channel affinity by traditional and hologram qSAR methods. | Bioorganic & medicinal chemistry letters 20030818 |
| [Antihistaminics. Side effects also after raising dosage at the placebo level]. | MMW Fortschritte der Medizin 20030529 |
| Mizolastine in primary acquired cold urticaria. | Journal of the American Academy of Dermatology 20030401 |
| Inhibition of mediator and cytokine release from dispersed nasal polyp cells by mizolastine. | Allergy 20021101 |
| Mizolastine provides effective symptom relief in patients suffering from perennial allergic rhinitis: a double-blind, placebo-controlled study versus loratadine. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20020901 |
| Comparative activity of cetirizine and mizolastine on histamine-induced skin wheal and flare responses at 24 h. | British journal of clinical pharmacology 20020301 |
| A double-blind, randomized, single-dose, crossover comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjects. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20020201 |
| [Hepatotoxicity of mizolastine (Mizollen) : report of 2 cases]. | Gastroenterologie clinique et biologique 20011201 |
| UV erythema reducing capacity of mizolastine compared to acetylsalicylic acid or both combined in comparison to indomethacin. | Photochemistry and photobiology 20011001 |
| Mizolastine in the treatment of seasonal allergic rhinoconjunctivitis: a European clinical experience with 5408 patients managed in daily practice (PANEOS SAR Study). | Allergy 20010701 |
| Population pharmacokinetic analysis and optimization of the experimental design for mizolastine solution in children. | Journal of pharmacokinetics and pharmacodynamics 20010601 |
| One-year treatment of chronic urticaria with mizolastine: efficacy and safety. J Eur Acad Dermatol Venereol 2000; 14: 83-90. | Journal of the European Academy of Dermatology and Venereology : JEADV 20010101 |
| Clinical pharmacokinetics of mizolastine. | Clinical pharmacokinetics 20010101 |
| Inhibition of HERG1 K(+) channels by the novel second-generation antihistamine mizolastine. | British journal of pharmacology 20001101 |
| Antiallergic effects of H1-receptor antagonists. | Allergy 20000101 |
| Efficacy and safety of mizolastine 10 mg in a placebo-controlled comparison with loratadine in chronic idiopathic urticaria: results of the MILOR Study. | Journal of the European Academy of Dermatology and Venereology : JEADV 19990101 |
| Mizolastine: a review of its use in allergic rhinitis and chronic idiopathic urticaria. | BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy 19980701 |