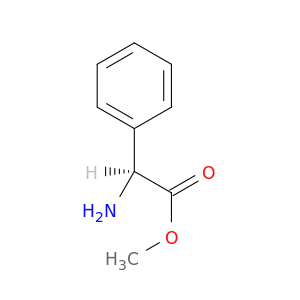
(S)-Methyl 2-amino-2-phenylacetate
| Title | Journal |
|---|---|
| Life cycle assessment of solar photo-Fenton and solar photoelectro-Fenton processes used for the degradation of aqueous α-methylphenylglycine. | Journal of environmental monitoring : JEM 20110101 |
| Functionalization of methyl (R)-phenylglycinate through orthopalladation: C-Hal, C-O, C-N, and C-C bond coupling. | Inorganic chemistry 20091221 |
| NMDA receptor affinities of 1,2-diphenylethylamine and 1-(1,2-diphenylethyl)piperidine enantiomers and of related compounds. | Bioorganic & medicinal chemistry 20090501 |
| Coupling solar photo-Fenton and biotreatment at industrial scale: main results of a demonstration plant. | Journal of hazardous materials 20070731 |
| Life cycle assessment of a coupled solar photocatalytic-biological process for wastewater treatment. | Water research 20061101 |
| Efficient enantioselective hydrolysis of D,L-phenylglycine methyl ester catalyzed by immobilized Candida antarctica lipase B in ionic liquid containing systems. | Journal of biotechnology 20060820 |
| Amine degradation by 4,5-epoxy-2-decenal in model systems. | Journal of agricultural and food chemistry 20060322 |
| Stereoselective acylation of a racemic amine with C(alpha)-methyl phenylglycine-based dipeptide 5(4H)-oxazolones. | Chirality 20051001 |
| Determination of the absolute configuration of Anisotome irregular diterpenes: application of CD and NMR methods. | Chirality 20041001 |
| Increasing the synthetic performance of penicillin acylase PAS2 by structure-inspired semi-random mutagenesis. | Protein engineering, design & selection : PEDS 20040701 |
| A novel chiral terpyridine macrocycle as a fluorescent sensor for enantioselective recognition of amino acid derivatives. | Chemical communications (Cambridge, England) 20040221 |
| Lipase-catalysed enantioselective ammonolysis of phenylglycine methyl ester in organic solvent. | Biotechnology and applied biochemistry 20031001 |
| Enhanced enzymatic synthesis of a semi-synthetic cephalosprin, cefaclor, with in situ product removal. | Biotechnology letters 20030701 |
| Improving lipase-catalyzed enantioselective ammonolysis of phenylglycine methyl ester in organic solvent by in situ racemization. | Biotechnology letters 20030301 |
| [Three dimensional structure analysis of organic chemical compounds from natural sources using NMR spectral analysis]. | Kokuritsu Iyakuhin Shokuhin Eisei Kenkyujo hokoku = Bulletin of National Institute of Health Sciences 20030101 |
| [Lipase-catalyzed enantioselective ammonolysis of racemic phenylglycine methyl ester in organic solvent]. | Sheng wu gong cheng xue bao = Chinese journal of biotechnology 20020101 |
| Biotransformations catalyzed by multimeric enzymes: stabilization of tetrameric ampicillin acylase permits the optimization of ampicillin synthesis under dissociation conditions. | Biomacromolecules 20010101 |