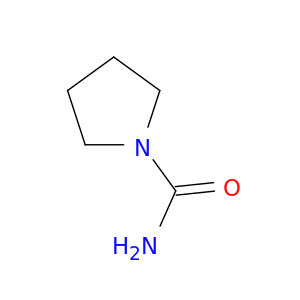
Pyrrolidine-1-carboxylic acid amide
| Title | Journal |
|---|---|
| Biophysical and cellular-uptake properties of mixed-sequence pyrrolidine-amide oligonucleotide mimics. | Chemistry (Weinheim an der Bergstrasse, Germany) 20111216 |
| A drug targeting only p110α can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types. | The Biochemical journal 20110815 |
| Neighboring pyrrolidine amide participation in thioether oxidation. Methionine as a 'hopping' site. | Organic letters 20110603 |
| Antidepressant potential of nitrogen-containing heterocyclic moieties: An updated review. | Journal of pharmacy & bioallied sciences 20110101 |
| Structural insights into substrate specificity in variants of N-acetylneuraminic Acid lyase produced by directed evolution. | Journal of molecular biology 20101119 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| The development and SAR of pyrrolidine carboxamide 11beta-HSD1 inhibitors. | Bioorganic & medicinal chemistry letters 20100501 |
| Discovery of potential new InhA direct inhibitors based on pharmacophore and 3D-QSAR analysis followed by in silico screening. | European journal of medicinal chemistry 20090901 |
| Drug discovery using chemical systems biology: repositioning the safe medicine Comtan to treat multi-drug and extensively drug resistant tuberculosis. | PLoS computational biology 20090701 |
| CoMFA based de novo design of pyrrolidine carboxamides as inhibitors of enoyl acyl carrier protein reductase from Mycobacterium tuberculosis. | Journal of molecular modeling 20081001 |
| Cyclobutane-containing alkaloids: origin, synthesis, and biological activities. | The open medicinal chemistry journal 20080101 |
| Stereospecific backbone methylation of pyrrolidine-amide oligonucleotide mimics (POM). | Chemical communications (Cambridge, England) 20060407 |
| Synthesis of the 1,6,8-trioxadispiro[4.1.5.2]tetradec-11-ene ring system present in the spirolide family of shellfish toxins and its conversion into a 1,6,8-trioxadispiro[4.1.5.2]-tetradec-9-en-12-ol via base-induced rearrangement of an epoxide. | Organic & biomolecular chemistry 20041221 |
| Synthesis and antibacterial evaluation of 1beta-methyl-2-(5-substituted heterocyclic carbamoyl)pyrrolidin-3-ylthio)carbapenem derivatives. | Archiv der Pharmazie 20040701 |
| Synthesis and absolute configuration of MQ-A3 [1-(14'-methylhexadecanoyl) pyrrolidine], a novel aliphatic pyrrolidine amide from the tropical convolvulaceous species. | Bioscience, biotechnology, and biochemistry 20010201 |
| Kinetically selective binding of single stranded RNA over DNA by a pyrrolidine-amide oligonucleotide mimic (POM). | Nucleosides, nucleotides & nucleic acids 20010101 |