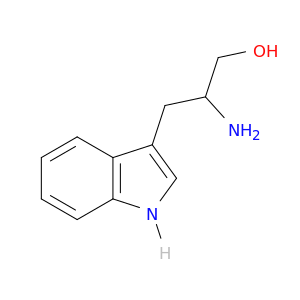
beta-amino-1H-indole-3-propanol
| Title | Journal |
|---|---|
| Quantifying prefibrillar amyloids in vitro by using a 'thioflavin-like' spectroscopic method. | Chembiochem : a European journal of chemical biology 20100903 |
| Amino acid deprivation links BLIMP-1 to the immunomodulatory enzyme indoleamine 2,3-dioxygenase. | Journal of immunology (Baltimore, Md. : 1950) 20091101 |
| Enantioselective formal synthesis of (+)-dihydrocorynantheine and (-)-dihydrocorynantheol. | The Journal of organic chemistry 20090206 |
| Direct high-performance liquid chromatographic separation of the enantiomers of an aromatic amine and four aminoalcohols using polysaccharide chiral stationary phases and acidic additive. | Chirality 20070801 |
| Straightforward methodology for the enantioselective synthesis of benzo[a]- and indolo[2,3-a]quinolizidines. | The Journal of organic chemistry 20070706 |