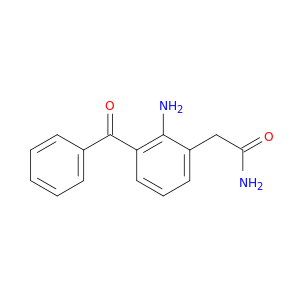
2-(2-amino-3-benzoylphenyl)acetamide
| Title | Journal |
|---|---|
| Nepafenac: an ophthalmic nonsteroidal antiinflammatory drug for pain after cataract surgery. | The Annals of pharmacotherapy 20130601 |
| Prophylactic nepafenac and ketorolac versus placebo in preventing postoperative macular edema after uneventful phacoemulsification. | Journal of cataract and refractive surgery 20120901 |
| Role of topical nepafenac in prevention and treatment of macular edema after vitreoretinal surgery. | Retina (Philadelphia, Pa.) 20120201 |
| [Phase III open-label study of nepafenac ophthalmic suspension 0.1% for inflammation and ocular pain following ophthalmic surgery]. | Nippon Ganka Gakkai zasshi 20120201 |
| Prevention of post cataract-surgery cystoid macular edema with nepafenac. | Journal of cataract and refractive surgery 20120101 |
| Phototherapeutic keratectomy. | Indian journal of ophthalmology 20120101 |
| Emerging pharmacotherapies for diabetic macular edema. | Experimental diabetes research 20120101 |
| Update on twice-daily bromfenac sodium sesquihydrate to treat postoperative ocular inflammation following cataract extraction. | Clinical ophthalmology (Auckland, N.Z.) 20120101 |
| Topical steroid and non-steroidal anti-inflammatory drugs inhibit inflammatory cytokine expression on the ocular surface in the botulinum toxin B-induced murine dry eye model. | Molecular vision 20120101 |
| Comparison of bromfenac 0.09% QD to nepafenac 0.1% TID after cataract surgery: pilot evaluation of visual acuity, macular volume, and retinal thickness at a single site. | Clinical ophthalmology (Auckland, N.Z.) 20120101 |
| Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: a randomized trial. | Indian journal of ophthalmology 20120101 |
| Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy. | Clinical ophthalmology (Auckland, N.Z.) 20120101 |
| Phase II placebo-controlled study of nepafenac ophthalmic suspension 0.1% for postoperative inflammation and ocular pain associated with cataract surgery in Japanese patients. | Journal of ophthalmic inflammation and infection 20111201 |
| A randomized comparison of to-aqueous penetration of ketorolac 0.45%, bromfenac 0.09% and nepafenac 0.1% in cataract patients undergoing phacoemulsification. | Current medical research and opinion 20111201 |
| Prostaglandin E2 inhibition of ketorolac 0.45%, bromfenac 0.09%, and nepafenac 0.1% in patients undergoing phacoemulsification. | Advances in therapy 20111201 |
| Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery. | Journal of cataract and refractive surgery 20110901 |
| Nepafenac for epiretinal membrane surgery. | Ophthalmology 20110701 |
| Ex vivo corneal epithelial wound healing following exposure to ophthalmic nonsteroidal anti-inflammatory drugs. | Clinical ophthalmology (Auckland, N.Z.) 20110101 |
| Critical appraisal of ophthalmic ketorolac in treatment of pain and inflammation following cataract surgery. | Clinical ophthalmology (Auckland, N.Z.) 20110101 |
| Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. | PloS one 20110101 |
| Evaluation of analgesic efficacy of bromfenac sodium ophthalmic solution 0.09% versus ketorolac tromethamine ophthalmic solution 0.5% following LASEK or Epi-LASIK. | Clinical ophthalmology (Auckland, N.Z.) 20110101 |
| Potential Therapeutic Roles for Inhibition of the PI3K/Akt/mTOR Pathway in the Pathophysiology of Diabetic Retinopathy. | Journal of ophthalmology 20110101 |
| Beneficial effects of a novel RAGE inhibitor on early diabetic retinopathy and tactile allodynia. | Molecular vision 20110101 |
| [Progresses in antiinflamatory treatment in cataract surgery]. | Oftalmologia (Bucharest, Romania : 1990) 20110101 |
| Combination therapies in ophthalmology: implications for intravitreal delivery. | Journal of ophthalmic & vision research 20110101 |
| Nepafenac-assisted mydriasis in a rabbit model. | Journal of cataract and refractive surgery 20101001 |
| Topical nepafenac for treatment of exudative age-related macular degeneration. | Acta ophthalmologica 20100301 |
| The effects of nepafenac and amfenac on retinal angiogenesis. | Brain research bulletin 20100215 |
| NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. | Retina (Philadelphia, Pa.) 20100201 |
| Impact of nepafenac 0.1% on macular thickness and postoperative visual acuity after cataract surgery in patients at low risk for cystoid macular oedema. | Eye (London, England) 20100101 |
| Diabetic cataract-pathogenesis, epidemiology and treatment. | Journal of ophthalmology 20100101 |
| Comparison of three strains of diabetic rats with respect to the rate at which retinopathy and tactile allodynia develop. | Molecular vision 20100101 |
| Inflammatory mediators and angiogenic factors in choroidal neovascularization: pathogenetic interactions and therapeutic implications. | Mediators of inflammation 20100101 |
| Use of nepafenac (Nevanac) in combination with intravitreal anti-VEGF agents in the treatment of recalcitrant exudative macular degeneration requiring monthly injections. | Clinical ophthalmology (Auckland, N.Z.) 20100101 |
| Ocular pharmacokinetics of 0.45% ketorolac tromethamine. | Clinical ophthalmology (Auckland, N.Z.) 20100101 |
| Differential effects of non-steroidal anti-inflammatory drugs on mitochondrial dysfunction during oxidative stress. | Archives of biochemistry and biophysics 20091001 |
| Vitreous nonsteroidal antiinflammatory drug concentrations and prostaglandin E2 levels in vitrectomy patients treated with ketorolac 0.4%, bromfenac 0.09%, and nepafenac 0.1%. | Retina (Philadelphia, Pa.) 20091001 |
| Nepafenac-associated bilateral corneal melt after photorefractive keratectomy. | Cornea 20090901 |
| Nepafenac dosing frequency for ocular pain and inflammation associated with cataract surgery. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20090801 |
| Nepafenac: new drug. After cataract surgery: just another NSAID eye drop. No better than other NSAID eye drops, and less convenient to use. | Prescrire international 20090801 |
| Gateways to clinical trials. | Methods and findings in experimental and clinical pharmacology 20090401 |
| Treatment of cystoid macular edema with the new-generation NSAID nepafenac 0.1%. | Clinical ophthalmology (Auckland, N.Z.) 20090101 |
| Management of ocular inflammation and pain following cataract surgery: focus on bromfenac ophthalmic solution. | Clinical ophthalmology (Auckland, N.Z.) 20090101 |
| Inhibition of surgically induced miosis and prevention of postoperative macular edema with nepafenac. | Clinical ophthalmology (Auckland, N.Z.) 20090101 |
| Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. | Clinical ophthalmology (Auckland, N.Z.) 20090101 |
| Increased neuronal nitric oxide synthase activity in retinal neurons in early diabetic retinopathy. | Molecular vision 20090101 |
| Etiology and treatment of the inflammatory causes of cystoid macular edema. | Journal of inflammation research 20090101 |
| Nepafenac dosing frequency for ocular pain and inflammation associated with cataract surgery. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20081201 |
| Topical nepafenac in the treatment of diabetic macular edema. | Clinical ophthalmology (Auckland, N.Z.) 20081201 |
| Effect of nepafenac sodium 0.1% on delayed corneal epithelial healing and haze after photorefractive keratectomy: retrospective comparative study. | Journal of cataract and refractive surgery 20080901 |
| Re: Pharmacokinetics and pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. | Journal of cataract and refractive surgery 20080801 |
| Topical ocular delivery of NSAIDs. | The AAPS journal 20080601 |
| Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension. | Clinical ophthalmology (Auckland, N.Z.) 20080601 |
| Amfenac increases the radiosensitivity of uveal melanoma cell lines. | Eye (London, England) 20080501 |
| Double-masked comparison of ketorolac tromethamine 0.4% versus nepafenac sodium 0.1% for postoperative healing rates and pain control in eyes undergoing surface ablation. | Cornea 20080401 |
| Double-masked comparison of ketorolac tromethamine 0.4% versus nepafenac sodium 0.1% for postoperative healing rates and pain control in eyes undergoing surface ablation. | Cornea 20080401 |
| Effects of topical nepafenac on corneal epithelial healing time and postoperative pain after PRK: a bilateral, prospective, randomized, masked trial. | Journal of refractive surgery (Thorofare, N.J. : 1995) 20080401 |
| Ketorolac versus nepafenac in cataract surgery. | Journal of cataract and refractive surgery 20080301 |
| Corneal melting after use of nepafenac in a patient with chronic cystoid macular edema after cataract surgery. | Eye & contact lens 20080301 |
| Topical nepafenac as an alternate treatment for cystoid macular edema in steroid responsive patients. | Retina (Philadelphia, Pa.) 20080101 |
| Cystoid and diabetic macular edema treated with nepafenac 0.1%. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20071201 |
| Re: Prostaglandin E(2) inhibition and aqueous concentration of ketorolac 0.4% and nepafenac 0.1% in patients undergoing phacoemulsification. | American journal of ophthalmology 20071201 |
| Analgesic and anti-inflammatory effectiveness of nepafenac 0.1% for cataract surgery. | Clinical ophthalmology (Auckland, N.Z.) 20071201 |
| Ketorolac tromethamine LS 0.4% versus nepafenac 0.1% in patients having cataract surgery. Prospective randomized double-masked clinical trial. | Journal of cataract and refractive surgery 20071101 |
| Nepafenac-associated corneal melt. | Journal of cataract and refractive surgery 20071101 |
| The use of a cyclooxygenase-2 inhibitor (Nepafenac) in an ocular and metastatic animal model of uveal melanoma. | Carcinogenesis 20070901 |
| In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. | Journal of cataract and refractive surgery 20070901 |
| Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. | Journal of cataract and refractive surgery 20070901 |
| Case of corneal melting associated with the use of topical nepafenac. | Cornea 20070901 |
| Double-masked comparison of ketorolac tromethamine 0.4% versus nepafenac sodium 0.1% for postoperative healing rates and pain control in eyes undergoing surface ablation. | Cornea 20070701 |
| Prostaglandin E2 inhibition and aqueous concentration of ketorolac 0.4% (acular LS) and nepafenac 0.1% (nevanac) in patients undergoing phacoemulsification. | American journal of ophthalmology 20070701 |
| Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. | Diabetes 20070201 |
| Effects of topical anti-inflammatory agents in a botulinum toxin B-induced mouse model of keratoconjunctivitis sicca. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20070201 |
| Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. | Journal of cataract and refractive surgery 20070101 |
| Comparative effects of the nonsteroidal anti-inflammatory drug nepafenac on corneal sensory nerve fibers responding to chemical irritation. | Investigative ophthalmology & visual science 20070101 |
| Double-masked study of the effects of nepafenac 0.1% and ketorolac 0.4% on corneal epithelial wound healing and pain after photorefractive keratectomy. | Advances in therapy 20070101 |
| The effects of a cyclooxygenase-2 (COX-2) expression and inhibition on human uveal melanoma cell proliferation and macrophage nitric oxide production. | Journal of carcinogenesis 20070101 |
| Effects of nonsteroidal ophthalmic drops on epithelial healing and pain in patients undergoing bilateral photorefractive keratectomy (PRK). | Advances in therapy 20070101 |
| Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. | Experimental diabetes research 20070101 |
| New drugs 06, part II. | Nursing 20060801 |
| The effect of a selective cyclooxygenase-2 (COX-2) inhibitor on the proliferation rate of retinoblastoma cell lines. | Eye (London, England) 20060501 |
| Comparison of the analgesic efficacy and safety of nepafenac ophthalmic suspension compared with diclofenac ophthalmic solution for ocular pain and photophobia after excimer laser surgery: a phase II, randomized, double-masked trial. | Clinical therapeutics 20060401 |
| Ocular permeation and inhibition of retinal inflammation: an examination of data and expert opinion on the clinical utility of nepafenac. | Current medical research and opinion 20060201 |
| New drugs: ramelteon, tipranavir, nepafenac, and deferasirox. | Journal of the American Pharmacists Association : JAPhA 20060101 |
| Nepafenac: a unique nonsteroidal prodrug. | International ophthalmology clinics 20060101 |
| Inflammation-mediated retinal edema in the rabbit is inhibited by topical nepafenac. | Inflammation 20031001 |
| Pharmacokinetics of topical ocular drug delivery: potential uses for the treatment of diseases of the posterior segment and beyond. | Current drug metabolism 20030601 |
| Topical nepafenac inhibits ocular neovascularization. | Investigative ophthalmology & visual science 20030101 |
| Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. | Inflammation 20000801 |
| Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. | Inflammation 20000801 |