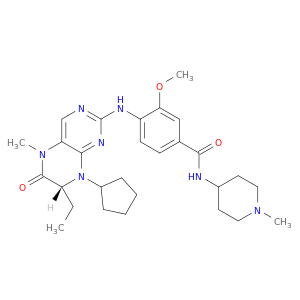
4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-7H-pteridin-2-yl]amino}-3-methoxy-N-(1-methylpiperidin-4-yl)benzamide
| Title | Journal |
|---|---|
| Eribulin synergizes with Polo-like kinase 1 inhibitors to induce apoptosis in rhabdomyosarcoma. | Cancer letters 20150828 |
| Centmitor-1, a novel acridinyl-acetohydrazide, possesses similar molecular interaction field and antimitotic cellular phenotype as rigosertib, on 01910.Na. | Molecular cancer therapeutics 20140501 |
| Dual kinase-bromodomain inhibitors for rationally designed polypharmacology. | Nature chemical biology 20140401 |
| BRD4 associates with p53 in DNMT3A-mutated leukemia cells and is implicated in apoptosis by the bromodomain inhibitor JQ1. | Cancer medicine 20131201 |
| Identification of potent Yes1 kinase inhibitors using a library screening approach. | Bioorganic & medicinal chemistry letters 20130801 |
| Hit to Lead optimization of a novel class of squarate-containing polo-like kinases inhibitors. | Bioorganic & medicinal chemistry letters 20121215 |
| Enabling and disabling polo-like kinase 1 inhibition through chemical genetics. | ACS chemical biology 20120615 |
| Using transcriptome sequencing to identify mechanisms of drug action and resistance. | Nature chemical biology 20120301 |
| Small interfering RNA library screen identified polo-like kinase-1 (PLK1) as a potential therapeutic target for breast cancer that uniquely eliminates tumor-initiating cells. | Breast cancer research : BCR 20120101 |
| Polo-like kinase 1 (PLK1) inhibition suppresses cell growth and enhances radiation sensitivity in medulloblastoma cells. | BMC cancer 20120101 |
| Comprehensive analysis of kinase inhibitor selectivity. | Nature biotechnology 20111030 |
| Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. | Nature cell biology 20111001 |
| Polo-like kinase 1 inhibition as a new therapeutic modality in therapy of cholangiocarcinoma. | Anticancer research 20111001 |
| Phosphorylation of Ataxin-10 by polo-like kinase 1 is required for cytokinesis. | Cell cycle (Georgetown, Tex.) 20110901 |
| Polo-like kinase-1 as a novel target in neoplastic mast cells: demonstration of growth-inhibitory effects of small interfering RNA and the Polo-like kinase-1 targeting drug BI 2536. | Haematologica 20110501 |
| Small molecule kinase inhibitor screen identifies polo-like kinase 1 as a target for neuroblastoma tumor-initiating cells. | Cancer research 20110215 |
| Microenvironmental influence on pre-clinical activity of polo-like kinase inhibition in multiple myeloma: implications for clinical translation. | PloS one 20110101 |
| Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. | Chemistry & biology 20101124 |
| Prediction of neutropenia-related effects of a new combination therapy with the anticancer drugs BI 2536 (a Plk1 inhibitor) and pemetrexed. | Clinical pharmacology and therapeutics 20101101 |
| Polo-like kinase 1 (Plk1) as a novel drug target in chronic myeloid leukemia: overriding imatinib resistance with the Plk1 inhibitor BI 2536. | Cancer research 20100215 |
| Treatment of biliary tract cancer with NVP-AEW541: mechanisms of action and resistance. | World journal of gastroenterology 20100114 |
| The Plk1 inhibitor BI 2536 temporarily arrests primary cardiac fibroblasts in mitosis and generates aneuploidy in vitro. | PloS one 20100101 |
| PLK1 down-regulates parainfluenza virus 5 gene expression. | PLoS pathogens 20090701 |
| Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. | The oncologist 20090601 |
| Identification of Polo-like kinase 1 as a potential therapeutic target in anaplastic thyroid carcinoma. | Cancer research 20090301 |
| Selectivity-determining residues in Plk1. | Chemical biology & drug design 20071201 |
| Pharmacological and functional comparison of the polo-like kinase family: insight into inhibitor and substrate specificity. | Biochemistry 20070821 |
| BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. | Current biology : CB 20070220 |