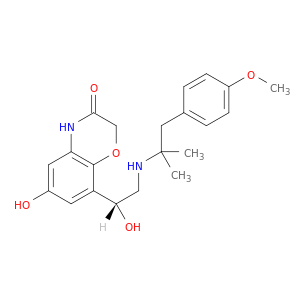
6-Hydroxy-8-[(1R)-1-hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]amino]ethyl]-2H-1,4-benzoxazin-3(4H)-one
| Title | Journal |
|---|---|
| Olodaterol: a review of its use in chronic obstructive pulmonary disease. | Drugs 20150401 |
| Dual regulation of β2-adrenoceptor messenger RNA expression in human lung fibroblasts by β2-cAMP signaling; delayed upregulated inhibitors oppose a rapid in onset, direct stimulation of gene expression. | Naunyn-Schmiedeberg's archives of pharmacology 20140101 |
| Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. | International journal of chronic obstructive pulmonary disease 20140101 |
| Olodaterol: first global approval. | Drugs 20131101 |
| Endothelin-1 enhances β₂-adrenoceptor gene transcription in human lung fibroblasts. | Life sciences 20121015 |
| β₂-adrenoceptors and muscarinic receptors mediate opposing effects on endothelin-1 expression in human lung fibroblasts. | European journal of pharmacology 20120915 |
| β₂ long-acting and anticholinergic drugs control TGF-β1-mediated neutrophilic inflammation in COPD. | Biochimica et biophysica acta 20120701 |
| Functional and biochemical rationales for the 24-hour-long duration of action of olodaterol. | The Journal of pharmacology and experimental therapeutics 20110601 |
| Pharmacological characterization of olodaterol, a novel inhaled beta2-adrenoceptor agonist exerting a 24-hour-long duration of action in preclinical models. | The Journal of pharmacology and experimental therapeutics 20100701 |
| Discovery of olodaterol, a novel inhaled beta2-adrenoceptor agonist with a 24 h bronchodilatory efficacy. | Bioorganic & medicinal chemistry letters 20100215 |