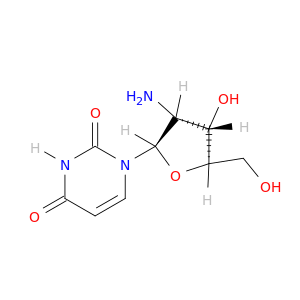
Uridine, 2'-amino-2'-deoxy-
| Title | Journal |
|---|---|
| S-Adenosylhomocysteine hydrolase of the protozoan parasite Trichomonas vaginalis: potent inhibitory activity of 9-(2-deoxy-2-fluoro-β,D-arabinofuranosyl)adenine. | Bioorganic & medicinal chemistry letters 20120615 |
| Porphyrin conjugated to DNA by a 2'-amido-2'-deoxyuridine linkage. | Bioorganic & medicinal chemistry letters 20080115 |
| Effective modulation of DNA duplex stability by reversible transition metal complex formation in the minor groove. | Journal of the American Chemical Society 20070801 |
| DNA and LNA oligonucleotides containing N2'-functionalised derivatives of 2'-amino-2'-deoxyuridine. | Bioorganic & medicinal chemistry letters 20060615 |
| [Monomers for oligonucleotide synthesis with linkers carrying reactive residues: II. Synthesis of phosphoamidites based of uridine and cytosine and containing a linker with methoxyoxalamide groups in position 2']. | Bioorganicheskaia khimiia 20040101 |
| Synthesis of 2'-modified oligodeoxynucleotides via on-column conjugation. | The Journal of organic chemistry 20010126 |
| Antiviral, antimetabolic and antineoplastic activities of 2'- or 3'-amino or -azido-substituted deoxyribonucleosides. | Biochemical pharmacology 19800615 |